Pathology News Roundup: August 31, 2021
The College of American Pathologists' (CAP) new evidence-based clinical practice guideline, “Laboratory Detection and Initial Diagnosis of...
The United States & Canadian Academy of Pathology (USCAP) has named Dr. Laura W. Lamps as its new president. Dr. Lamps is the Godfrey D. Stobbe Professor and Director of Gastrointestinal Pathology at the University of Michigan, where she has been on the faculty since 2017.

The College of American Pathologists (CAP) earned reapproval from the Centers for Medicare & Medicaid Services (CMS) as an accreditation organization for clinical laboratories. As reported in the Federal Register, the CMS determined the CAP, in its application for reapproval, met or exceeded the applicable Clinical Laboratory Improvement Amendments of 1988 (CLIA) requirements.
The CMS regulates all laboratory testing, except research, performed on humans in the U.S. through CLIA. It began granting deeming authority to accrediting organizations in 1994, with the CAP always winning renewals for six years, the maximum allowable period under federal regulation.
The CAP currently accredits nearly 8,000 laboratories worldwide and the program continues to grow despite the shrinking certificate of accreditation market of laboratories permitted to conduct moderate and/or high-complexity testing.
“Our accreditation program, grounded in peer-to-peer review, provides laboratories a practical and relevant inspection,” said CAP President Patrick Godbey, MD, FCAP, laboratory director, Southeastern Pathology Associates and Southeast Georgia Health System, Brunswick, Georgia. “We have the premier accreditation program because of the thousands of dedicated laboratory professionals, including CAP members, who volunteer to perform inspections. Our program offers an educational inspection process designed to continuously improve laboratories, safeguard patient testing, and provide the best patient care possible.”
As part of CAP accreditation, the CAP deploys teams to inspect on-site every two years, with self-inspection required between on-site visits. The teams assess compliance using extensive requirements detailed in CAP Accreditation Checklists.
“CAP accreditation checklist requirements are more complete and educational than other accreditors’ requirements,” said Richard M. Scanlan, MD, FCAP, medical director of the Oregon Health and Sciences University Hospital laboratory, Portland, Oregon. “With peer-based inspections, laboratories have a unique opportunity to learn from their peers and greater confidence in the thoroughness of their inspection to improve the quality of laboratory services.”

A team led by investigators at Weill Cornell Medicine and New York-Presbyterian has used advanced technology and analytics to map, at single-cell resolution, the cellular landscape of diseased lung tissue in severe COVID-19 and other infectious lung diseases.
In the study, published online March 29 in Nature, the researchers imaged autopsied lung tissue in a way that simultaneously highlighted dozens of molecular markers on cells. According to an article in the Cornell Chronicle, analyzing these data using novel analytical tools revealed new insights into the causes of damage in these lung illnesses and a rich data resource for further research.
“COVID-19 is a complex disease, and we still don’t understand exactly what it does to a lot of organs, but with this study we were able to develop a much clearer understanding of its effects on the lungs,” said co-senior author Dr. Olivier Elemento, professor of physiology and biophysics, director of the Caryl and Israel Englander Institute for Precision Medicine, associate director of the HRH Prince Alwaleed Bin Talal Bin Abdulaziz Alsaud Institute for Computational Biomedicine at Weill Cornell Medicine and co-Director of the WorldQuant Initiative for Quantitative Prediction, which funded the technology for single cell analysis of tissue. “I think the technological approach we used here is going to become standard for studying such diseases.”
Traditional tissue analysis, often using chemical stains or tagged antibodies that label different molecules on cells, can reveal important features of autopsied tissues. However, this approach is limited in the number of features it can mark simultaneously. It also usually doesn’t allow detailed analyses of individual cells in tissues while retaining information about where the cells were in the tissue.
The main technology the investigators employed in the study, a technology called imaging mass cytometry, largely overcomes those limitations. It uses a collection of metal-tagged antibodies that can simultaneously label up to several dozen molecular markers on cells within tissues. A special laser scans the labeled tissue sections, vaporizing the metal tags, and the metals’ distinct signatures are detected and correlated with the laser position. The technique essentially maps precisely where cells are in the sample as well as each cell’s surface receptors and other important identifying markers. Altogether over 650,000 cells were analyzed.
The researchers applied the method to 19 lung tissue samples autopsied from patients who had died of severe COVID-19, acute bacterial pneumonia, or bacterial or influenza-related acute respiratory distress syndrome, plus four lung tissue samples autopsied from people who had had no lung disease.
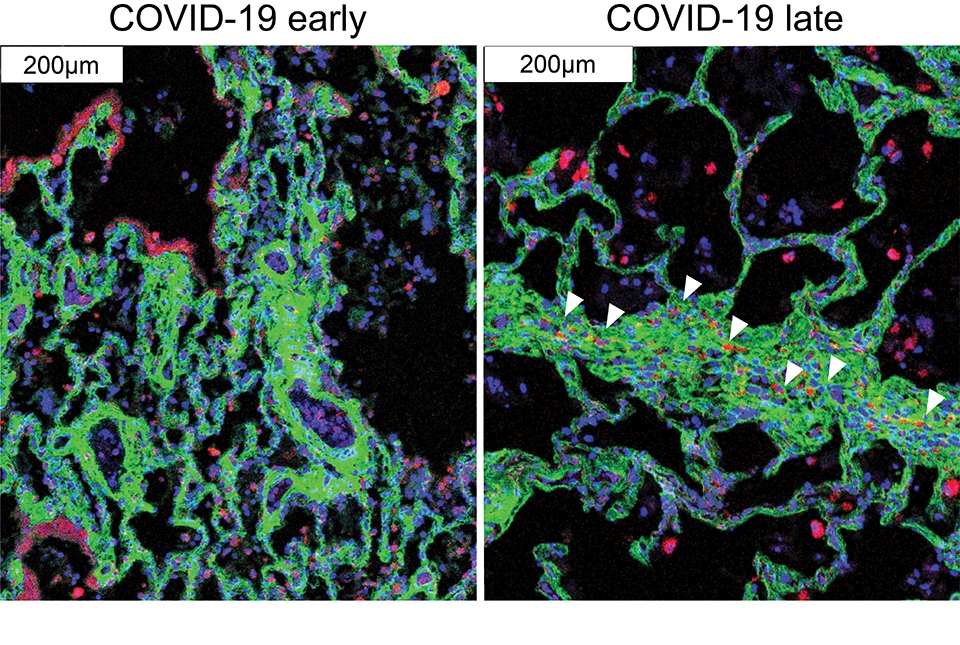 Immune cells (red) migrate near the cells that cause fibrosis (green) in late COVID-19. Images courtesy of André Rendeiro.
Immune cells (red) migrate near the cells that cause fibrosis (green) in late COVID-19. Images courtesy of André Rendeiro.
The findings in samples from COVID-19 cases were broadly consistent with what is known about the disease, but clarified this knowledge in much finer detail. They showed for example that cells called alveolar epithelial cells, which mediate the lungs’ gas-exchange function, are the main targets of infection by SARS-CoV-2, the coronavirus that causes COVID-19. The analysis suggested that these infected cells are not solely singled out for attack by lung-infiltrating immune cells, which may help explain why inflammation often keeps worsening in severe COVID-19 and ends up causing such extensive and relatively indiscriminate damage.
One surprise was that age and sex, two major factors in mortality risk for COVID-19, made no apparent difference at the histologic level, once COVID-19 had progressed to the severe stage.
The results also showed that white blood cells called macrophages are much more abundant in the lungs of severe COVID-19 patients compared to other lung diseases, whereas white blood cells called neutrophils are most prevalent in bacterial pneumonia—a distinction that may be relevant to the development of future treatments for these infectious diseases.

Voicebrook's Pathology News Roundup features industry headlines and insights that pathology professionals are talking about. Think we should be covering something in particular?
Send your suggestions to content@voicebrook.com
The College of American Pathologists' (CAP) new evidence-based clinical practice guideline, “Laboratory Detection and Initial Diagnosis of...
The College of American Pathologists (CAP) wraps up its annual meeting today. CAP21 “Advancing Medicine Today & Tomorrow” gave members the option to...
CAP Convinces HHS to Change COVID Reporting Rules The Department of Health and Human Services (HHS) responded to the College of American...